How an agency’s grasp on approval processes can make or break your medical content
If you work in medical affairs, you know that legal, medical, and regulatory review is crucial for the materials your team uses, whether internally, in the field, or at medical congress booths. But what about the agencies you partner with? Do they understand your company’s review process?
If the answer is no, the risks are longer timelines, higher costs, and potentially damaging your relationship with your reviewers. So, let’s take a look at how even agencies with the best intentions can inadvertently cause big headaches if they just don’t get how legal medical and regulatory review (LMR) is integral for the success of their project. And yes, different companies have different approval processes with different names and different platforms. We’ll use “LMR” and Veeva here for simplicity, given how ubiquitous they are in the industry.
LMR is not just the final hurdle
When an agency doesn’t understand your approval process, there are some obvious consequences that come to mind. They may not plan enough time for review, assuming that this will just need a day or two at the very end of the project. Or they may not understand how a different verdict—whether approved with changes or revise and resubmit—affects the next steps. But overall, there is one key mistake from which many others follow: an agency that doesn’t understand your company’s approval process will treat legal, medical, and regulatory review as an afterthought.
The dangers of waiting until the last minute
Treating LMR as an afterthought means that an agency may not be aware of how LMR plays a role not just at the end of a project but throughout it. For example, when putting together a slide deck, an agency may find it expedient to wait to double check the references they are citing until right before LMR submission. What’s the problem? Slide decks are often built from existing materials that may or may not be correct. Anyone working in the medical communications space knows they can’t just assume all the slides they’ve received as input for a project cite the correct references. This is especially true when the materials need to be uploaded in Veeva with references linked for LMR. If an agency just assumes the references are correct, it is very likely that when they upload to Veeva and start linking references, they will come across statements that aren’t actually covered by the references that are being cited. And when they do find these errors, they will need to either find a reference or rewrite content.
A related issue can arise when an agency puts off requesting references until the end of the project. Depending on the number of references and how your company handles purchasing and copyright clearance, it may take significant time to track down the references that need to be uploaded to Veeva. Waiting until the last minute to thoroughly check the references can substantially delay LMR and thus the date when your team can first use the material.
The wrong time for quality control
If an agency thinks of LMR as just the last step, they may not be proofreading carefully or running QC prior to submission. You can read more about the importance of QC for medical content development here. To be sure, not doing QC before Veeva upload creates more work on the agency’s side: when they catch a mistake while linking references in Veeva, they will need to go back to the source file, make the change, and re-upload. That may also involve re-linking anchors that don’t find the correct placement when the links are carried forward.
But more importantly, submitting materials that have copious typos and factual errors creates more work for reviewers and an environment of mistrust. If LMR reviewers get the impression that they can’t trust an agency’s attention to detail, it can make them feel that they are being asked to become proofreaders, double and triple checking every last footnote and reference to ensure that serious mistakes are not slipping through the cracks. That mistrust can then easily spread to other materials with the same project owner at your company (which could be you!). When reviewers know that you’re the project owner, you don’t want them thinking “Oh great, this person again. Last time we got something from their agency, it was full of mistakes.”
Misunderstanding who LMR are, what they do, and what they want
An agency should know that LMR are not there to proofread for grammar, spelling, and style. Of course, no one is perfect, and they may spot one or two small typos. But an agency needs to include time for a professional medical copyeditor in their budget and project plan, so that when it comes time for LMR, the material submitted is clean and polished.
However, far beyond any typo or misplaced reference citation, the single biggest mistake an agency can make is how they perceive LMR feedback. Do they view LMR comments as overly picky and critical? Do they think of LMR as existing only to split hairs and argue about the number of data points that fit on the head of a pin? If an agency perceives LMR as adversaries, they’re likely to build an adversarial relationship with them, and think in terms of “what they can get past LMR.”
This is bad for the project at hand, but it’s also bad for you. It means you may need to spend time monitoring an agency’s work closely to ensure they’re taking LMR feedback seriously, or repeatedly jumping in to overcome impasses that arise when an agency insists that they’re right and the feedback is wrong. Those probably aren’t the most valuable uses of your time. And moreover, you don’t want an agency with the wrong idea about LMR to damage your relationship with your company’s reviewers. That’s damage that can have a lasting impact on your career, since LMR are among the most important internal stakeholders for all your team’s medical communication initiatives.
What does it look like when you work with an agency that understands LMR?
The popularity of doomscrolling aside, to end on a positive note, let’s look at the flipside.
An agency that understands your approval process knows that legal, medical, and regulatory reviewers are crucial partners for the success of your initiatives. Most importantly, a good agency understands that LMR reviewers help keep patients safe, physicians properly informed, and your company’s risks minimized. That kind of agency is thankful that LMR is offering guidance to create better materials. And they seek to build but a collaborative rather than combative relationship with LMR.
Collaborating with LMR means anticipating their feedback based on experience working with them. To be sure, this involves nuts-and-bolts medical copyediting to include the correct legal disclaimers where necessary and avoid language that LMR has deemed too promotional in med comms materials in the past. But it also goes further, with the agency understanding the concerns behind the feedback that LMR shares, and proactively making suggestions that address those concerns.
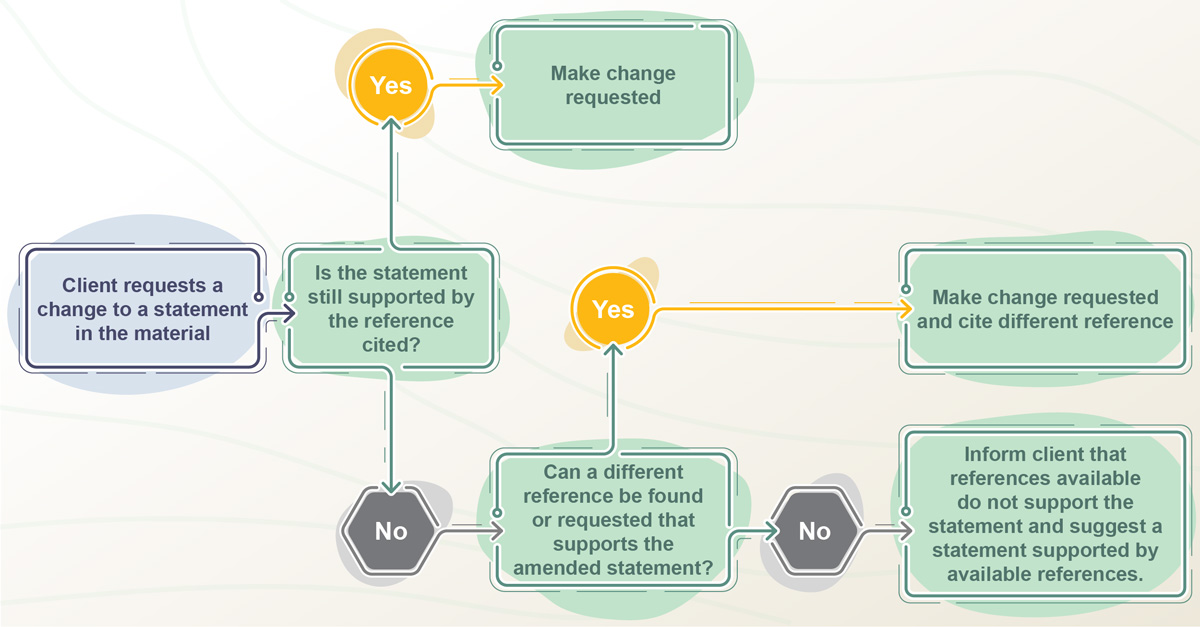
Instead of an afterthought, an agency with the right idea about LMR plans for review and approval at every step of their workflow. For example, during the early stages of a project, if someone on your team requests a change to a statement in a concept, the agency’s medical content developer checks to make sure the reference cited still supports the statement. If not, they check to see if the statement can be supported by any references they have access to. If not, they flag to you, to make sure that no statements are included in the material that can’t be substantiated. That means they are also aware whenever incorporating feedback if they need to request references and do so promptly instead of waiting until linking to anchors in Veeva.
The value of a collaborative relationship with LMR
Viewing LMR feedback as guidance that protects patients, healthcare professionals, and your company goes a long way in framing the approval process. Within that frame, the agency is not just following orders. The agency is an active partner, supporting reviewers with the information they need to critically assess the material under review. The agency makes suggestions that address not just the letter but also the spirit of the feedback. And in doing so, the agency helps you foster that collaborative relationship with your reviewers for the long haul.